Sep 1, 2021
Top COVID-19 News
Amid the pandemic, news is fast-moving – and sometimes confusing. Coverage is here to help. Our new series provides a clear, fact-based digest of the top news for health consumers.

Full approval for Pfizer
The FDA has granted full approval to Pfizer-BioNTech’s coronavirus vaccine for people 16 and older, making it the first vaccine to move beyond emergency use authorization status in the U.S. You may see the vaccine referred to by its brand name, Comirnaty.
What does that mean? Emergency use authorization provides access to life-saving medical products when there are no available options. The U.S.’s authorized vaccines had to undergo extensive testing and prove their benefits outweighed risks, preventing infection more than 50% of the time – a standard they easily surpassed. For full approval, the FDA launches a more detailed and rigorous process, including examining at least six months of data and inspecting manufacturing facilities. In this case, Pfizer presented the FDA with data from 44,000 clinical trial participants showing the vaccine was 91% effective in preventing infection. The Pfizer-BioNTech vaccine will continue to be authorized for emergency use for children ages 12-15 while Pfizer collects the necessary data required for full approval. Regulators are reviewing Moderna’s application for full approval and Johnson & Johnson is expected to apply soon.

Delta’s toll
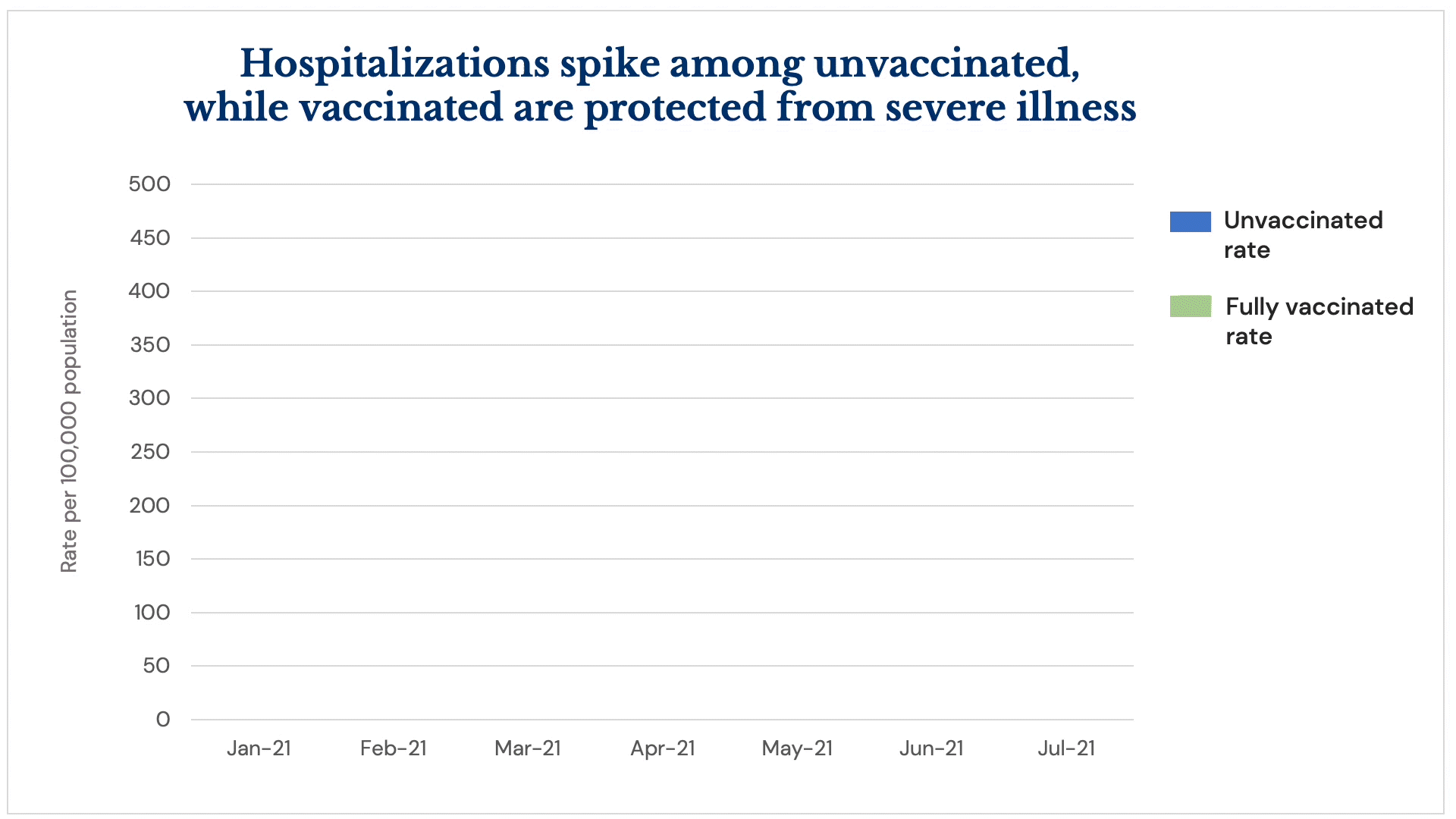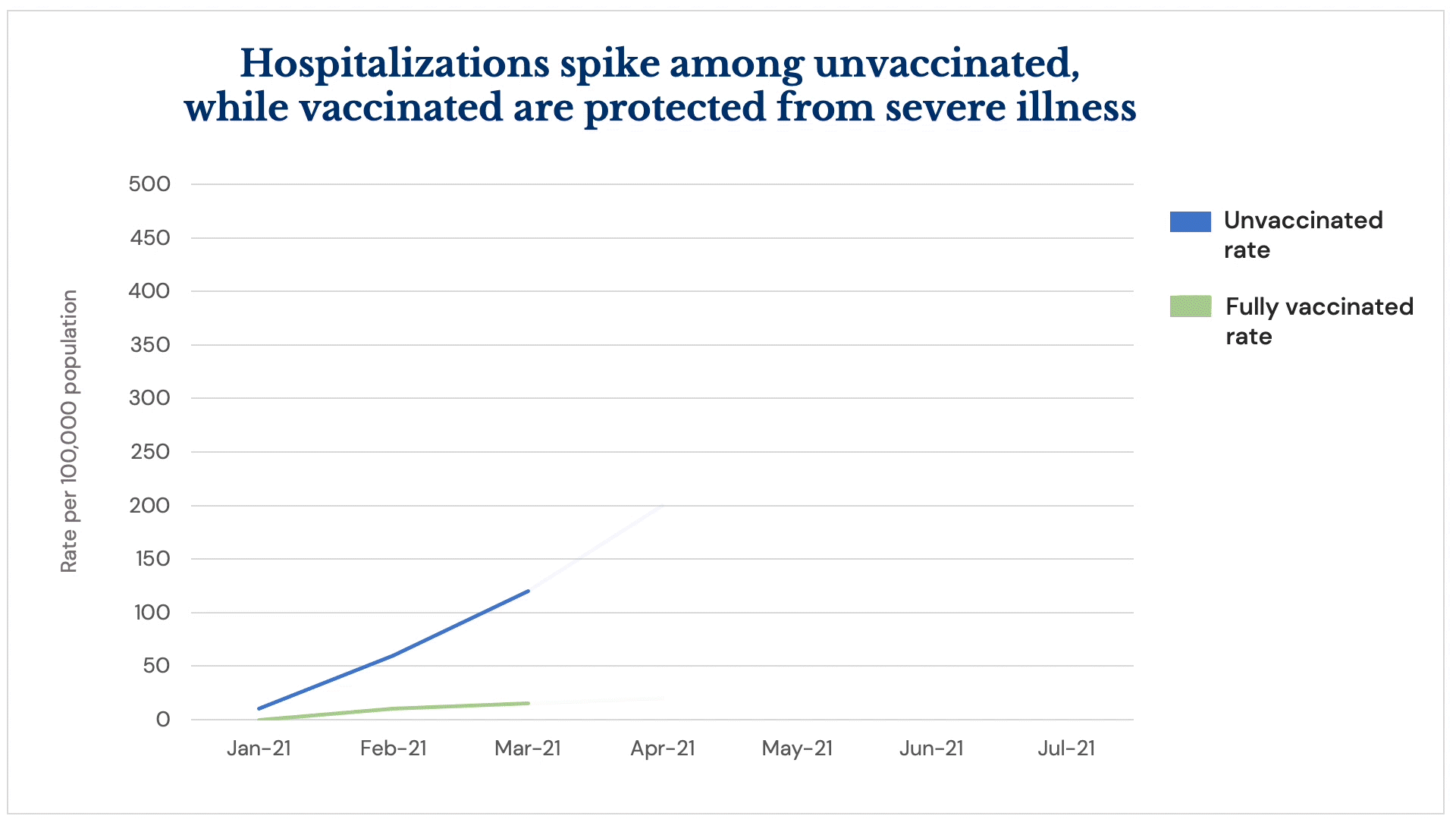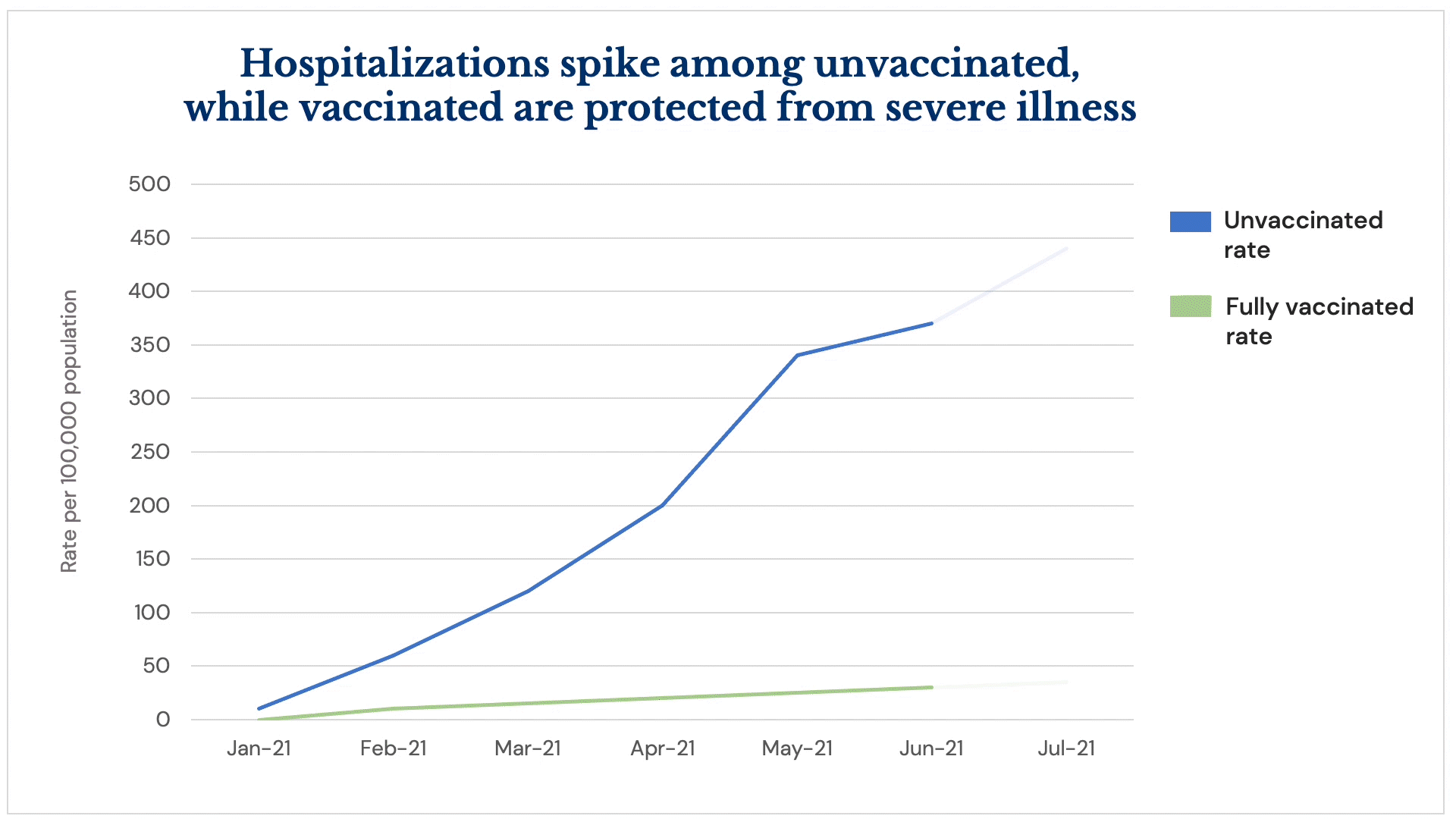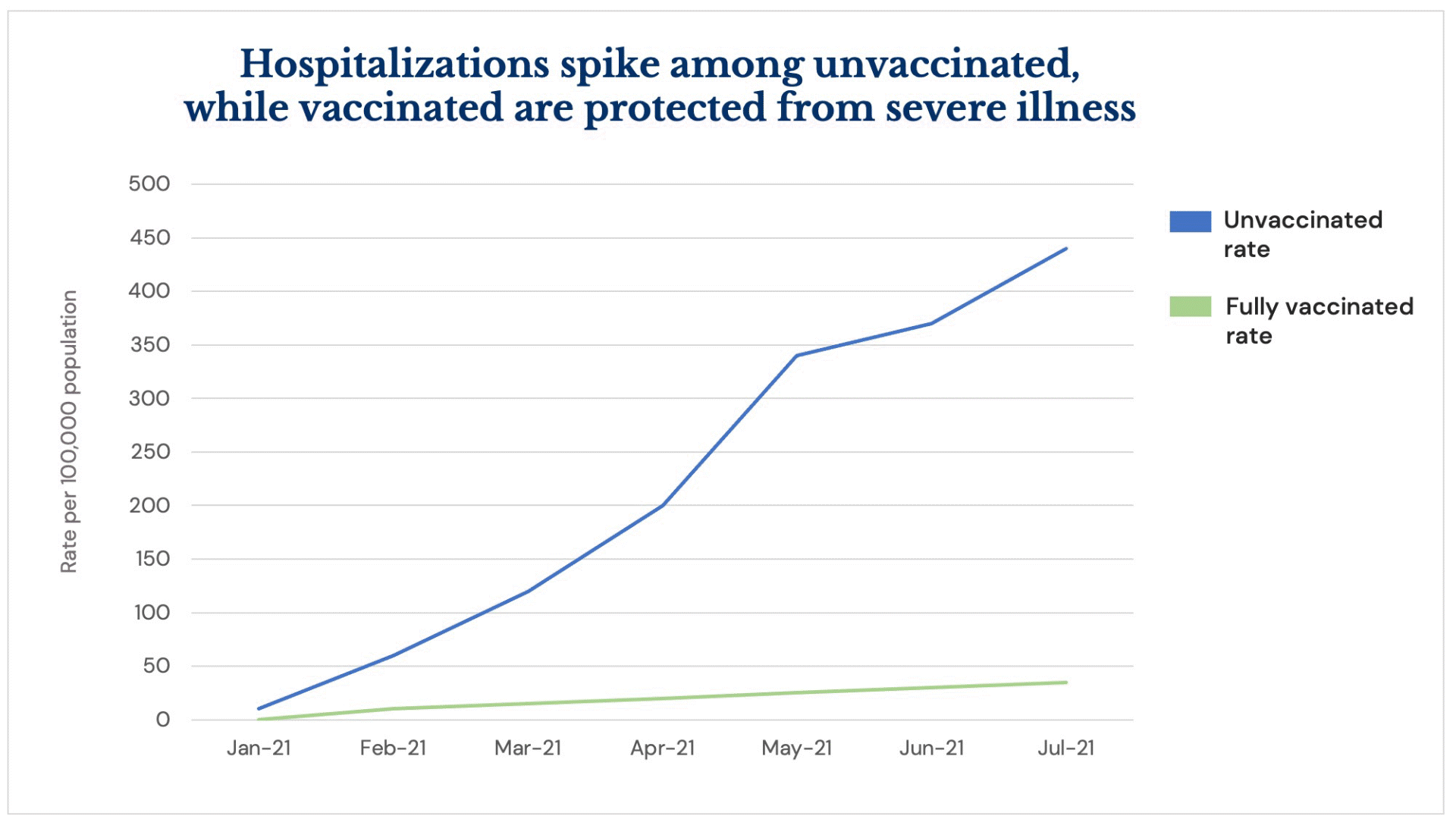
All three U.S. vaccines are continuing to prove highly effective at preventing the severe illness from COVID infection associated with hospitalization and death, but areas of the country with low vaccination rates are seeing a surge of hospitalizations amid the Delta variant’s fast spread.
How can we help? Vaccines are free and widely available to anyone 12 or over. To make an appointment or find a walk-up site, visit https://vaxfinder.mass.gov/. Because the virus can spread even from vaccinated people, the CDC recommends wearing a mask indoors in public in areas of substantial or high transmission, which currently includes almost the entire country, including all of Massachusetts. To help stem the spread, Massachusetts public school students and staff will be required to wear masks indoors in schools until at least Oct. 1, 2021. Many of the state’s cities and towns, including Boston, also have implemented indoor mask mandates for public settings.

Boosters for the immunocompromised
Some fully vaccinated immunocompromised people, such as those who are taking immunosuppressant medications, have had an organ or stem cell transplant, or are undergoing cancer treatments, can receive third Pfizer or Moderna shots to boost their immunity under new FDA authorization. “After a thorough review of the available data, the FDA determined that this small, vulnerable group may benefit from a third dose of the Pfizer-BioNTech or Moderna vaccines,” said Acting FDA Commissioner Janet Woodcock.
How about everyone else? The FDA and an independent panel of Centers for Disease Control and Prevention advisers are reviewing the data and will issue guidance based on the scientific evidence. If they approve boosters for other vaccinated people, the third shots will be offered first to people who received them earliest in the vaccine rollout, including health care workers and nursing home residents. (Researchers are still examining whether boosters will be needed for people who received the Johnson & Johnson vaccine as well.)

